Xiaowen Bai, MD, PhD
Professor
Locations
- Cell Biology, Neurobiology & Anatomy
Contact Information
Education
PhD, Stem Cell Center, Beijing University, Beijing, China, 2004
MD, Shanxi Medical University, Taiyuan, Shanxi, China, 1993
Biography
Research Areas of Interest
- Anesthetics
- Brain Damage, Chronic
- Cell- and Tissue-Based Therapy
- Diabetic Cardiomyopathies
- Disease Models, Animal
- Ethanol
- MicroRNAs
- Mitochondria
- Neurotoxicity Syndromes
- Organoids
- RNA, Long Noncoding
- Stem Cells
Research Experience
- Adipose Tissue
- Adult Stem Cells
- Brain
- Cell Differentiation
- Cell Survival
- Cell- and Tissue-Based Therapy
- Diabetic Cardiomyopathies
- Embryonic Stem Cells
- Gene Regulatory Networks
- Induced Pluripotent Stem Cells
- MicroRNAs
- Mitochondria
Leadership Positions
- 2022 - Present. Executive Director, Hematology of World Federation of Chinese Medicine Societies (WFCMS)
Research Interests
Research Area 1: Non-coding RNAs, mitochondria, and cell stress-related genes in neurodegeneration:
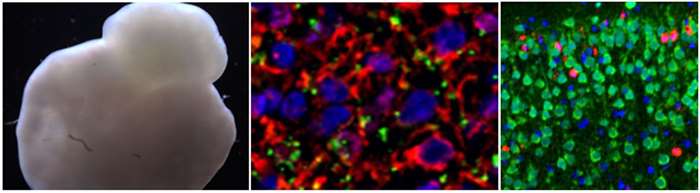
Neurological disorders have emerged as a predominant healthcare concern in recent years due to their severe consequences on quality of life and prevalence throughout the world. Understanding the underlying mechanisms of these diseases and the interactions between different brain cell types is essential for the development of new therapeutics. Many drugs (e.g., anesthetics), environmental factors (e.g., alcohol), diseases, and genetic risks are related to neurodegeneration. We examine the novel molecular mechanisms underlying the roles of microRNAs, long non-coding RNAs, mitochondria, immediate early and other cell stress-related genes in neurodegeneration using both mouse, and human stem cell-derived brain cell and three-dimensional mini brain models
Human induced pluripotent stem cell-derived mini brain (left) and the neurons in mini brain (middle). Alcohol-induced neuroapoptosis (red) in neonatal mouse brain (right).
Research Area 2: Stem cell-mediated myocardial regeneration
Myocardial infarction is one of the major causes of death throughout the world. Currently, there is not a highly effective approach for treatment. Stem cells hold promise in repairing injured cardiac tissue. Our lab is involved in studying the effect of the transplantation of adipose tissue-derived stem cells and induced pluripotent stem cell-derived cardiomyocytes on myocardial regeneration following ischemia injury. A molecular imaging method has been developed to investigate the molecular mechanisms controlling homing, engraftment, and survival of injected cells in vivo.
Research Area 3: The mechanisms of impaired cardioprotection under diabetic conditions
Hyperglycemia has been shown to be particularly detrimental to the cardioprotective effects, with the underlying mechanisms remaining largely unknown. We have developed and validated a clinically relevant model of functional human cardiomyocytes derived from both normal induced pluripotent stem cells (iPSCs) and diabetes mellitus iPSCs. This in vitro model of human disease will enable developmental and comparative studies of normal and diabetic cardiomyocytes to address genetic and environmental mechanisms responsible for attenuation of cardioprotection signaling in diabetics.
Functional contracting human cardiomyocytes generated from induced pluripotent stem cells
Mitochondrial dynamics (movement, fusion and fission) in human neurons generated from embryonic stem cells. Green signals represent mitochondria. Red and yellow colors highlight mitochondria in process of fusion.
Publications
-
SNRK regulates TGFβ levels in atria to control cardiac fibrosis.
(Thirugnanam K, Rizvi F, Jahangir A, Homar P, Shabnam F, Palecek SP, Kumar SN, Pan A, Bai X, Sekine H, Ramchandran R.) bioRxiv. 2024 Sep 26 PMID: 39386731 PMCID: PMC11463613 10/10/2024
-
(Bai X.) Neural Regen Res. 2024 May;19(5):953-954 PMID: 37862185 PMCID: PMC10749634 10/20/2023
-
(Pant T, Uche N, Juric M, Zielonka J, Bai X.) Redox Biol. 2024 Apr;70:103077 PMID: 38359749 PMCID: PMC10877431 SCOPUS ID: 2-s2.0-85185554817 02/16/2024
-
(Arzua T, Yan Y, Liu X, Dash RK, Liu QS, Bai X.) Transl Psychiatry. 2024 Jan 22;14(1):51 PMID: 38253552 PMCID: PMC10803756 SCOPUS ID: 2-s2.0-85182859098 01/23/2024
-
(Yan Y, Logan S, Liu X, Chen B, Jiang C, Arzua T, Ramchandran R, Liu QS, Bai X.) Cells. 2022 Aug 11;11(16) PMID: 36010580 PMCID: PMC9406780 SCOPUS ID: 2-s2.0-85136592621 08/27/2022
-
(Liu X, Yu H, Chen B, Friedman V, Mu L, Kelly TJ, Ruiz-Pérez G, Zhao L, Bai X, Hillard CJ, Liu QS.) Biomedicines. 2022 Jul 22;10(8) PMID: 35892676 PMCID: PMC9329798 SCOPUS ID: 2-s2.0-85137365256 07/28/2022
-
Identification of a dihydropyridine scaffold that blocks ryanodine receptors.
(Gunaratne GS, Rebbeck RT, McGurran LM, Yan Y, Arzua T, Frolkis T, Sprague DJ, Bai X, Cornea RL, Walseth TF, Marchant JS.) iScience. 2022 Jan 21;25(1):103706 PMID: 35059610 PMCID: PMC8760560 01/22/2022
-
(Yu H, Liu X, Chen B, Vickstrom CR, Friedman V, Kelly TJ, Bai X, Zhao L, Hillard CJ, Liu QS.) Cells. 2021 Dec 16;10(12) PMID: 34944056 PMCID: PMC8700250 SCOPUS ID: 2-s2.0-85121121427 12/25/2021
-
(Alt EU, Schmitz C, Bai X.) Cells. 2021 Sep 03;10(9) PMID: 34571951 PMCID: PMC8467324 SCOPUS ID: 2-s2.0-85115886729 09/29/2021
-
(Arzua T, Jiang C, Yan Y, Bai X.) Neurosci Biobehav Rev. 2021 Sep;128:633-647 PMID: 34186153 PMCID: PMC8357057 SCOPUS ID: 2-s2.0-85109839820 06/30/2021
-
(Jiang C, Arzua T, Yan Y, Bai X.) Int J Mol Sci. 2021 Jan 30;22(3) PMID: 33573239 PMCID: PMC7869012 SCOPUS ID: 2-s2.0-85100021031 02/13/2021
-
(Arzua T, Yan Y, Jiang C, Logan S, Allison RL, Wells C, Kumar SN, Schäfer R, Bai X.) Transl Psychiatry. 2020 Oct 13;10(1):347 PMID: 33051447 PMCID: PMC7553959 SCOPUS ID: 2-s2.0-85092448660 10/15/2020