Kelliher Laboratory Research Areas
Once thought to be a nutrient-rich environment protected from immune detection, we now appreciate that the cytosol is restrictive to non-adapted organisms. However, the mechanisms by which the mammalian cytosol kills microbial invaders remain largely uncharacterized. We investigate both the cytosolic defenses that target microbes in this environment, as well as the adaptations that professional cytosolic pathogens possess that enable them to survive and thrive in this restrictive compartment. We primarily use the cytosolic bacterium Listeria monocytogenes and macrophages to model these interactions.
One factor that is required for L. monocytogenes to survive in the cytosol of host cells is its penicillin-binding protein and serine/threonine-associated (PASTA) kinase PrkA. PASTA kinases are conserved in Gram-positive bacteria and are required for intrinsic resistance to cell envelope-targeting stressors, such as β-lactam antibiotics and host-derived factors like lysozyme. During cell wall stress, PrkA phosphorylates proteins involved in a variety of physiological processes, consistent with a role for PrkA as a global regulator in response to cell wall stress. We have found that one critical role of PrkA is to increase synthesis of the cell wall component peptidoglycan during stress and that this pathway is required for cytosolic survival. We are currently focused on understanding how other pathways modulated by PrkA promote adaptation to the host cytosol. In addition, given that PrkA is required for the virulence of a professional cytosolic pathogen, we are identifying and characterizing the host factor(s) that induce cell wall stress in cytosolic bacteria.
PASTA kinase signaling and virulence regulation in the cytosol: L. monocytogenes is a ubiquitous saprophyte that can become virulent in mammals by undergoing transcriptional reprogramming through its master virulence regulator PrfA, which is activated by multiple environmental cues. We have found that a ΔprkA mutant is defective in activating PrfA, suggesting that one or more PrkA substrates is involved in virulence activation in L. monocytogenes. We are exploring the hypothesis that cell wall stress sensed by PrkA is one environmental cue that promotes L. monocytogenes pathogenesis through PrfA activation and other pathways.
Crosstalk between PASTA kinase and other stress response systems in the cytosol: The cell envelope is a major target of innate host defenses (such as lysozyme and cationic antimicrobial peptides), and bacteria possess multiple cell envelope-sensing systems to sense and respond to cell wall attacks, including two-component systems (TCSs). Among the PrkA substrates identified in L. monocytogenes are two TCS sensor histidine kinases that are important during exposure to similar cell envelope-targeting stressors as PrkA. We are exploring the nexus of PASTA kinase and TCS signaling in L. monocytogenes, testing the hypothesis that PrkA integrates with cell envelope-responsive TCSs to optimize cell wall stress responses in the cytosol.
Identifying and characterizing cytosolic defenses: Given that the cell wall-sensing kinase PrkA is required for survival of L. monocytogenes in the cytosol, we hypothesize that there are cell wall-targeting host defenses in this environment. We aim to identify cell-autonomous defense mechanisms deployed against bacteria through orthogonal, host-directed proximity-dependent labeling and forward genetic screening platforms and use multidisciplinary approaches to characterize these defenses.
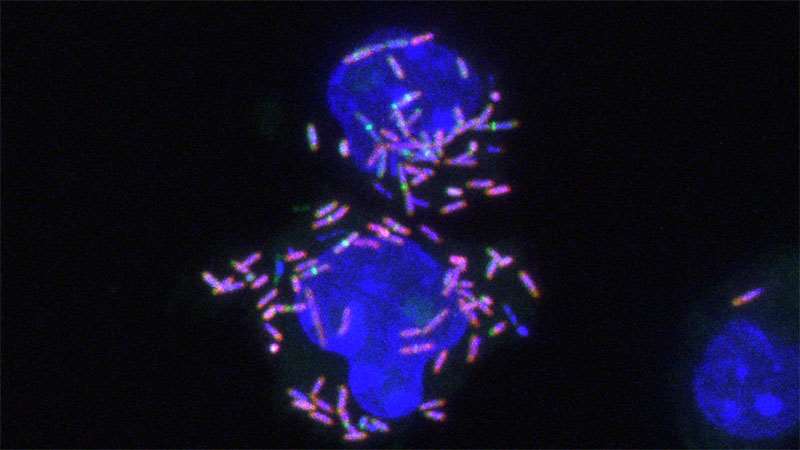
Figure 1. Macrophages infected with L. monocytogenes. DNA is labeled with DAPI (blue), bacteria are labeled with mCherry (magenta), and peptidoglycan is labeled with Alexa488 (green).

