
Dawn Wenzel, PhD
Assistant Professor
Locations
- TBRC C2950
Contact Information
General Interests
Education
BS, Biochemistry, University of Wisconsin-Madison, 2004
Biography
Dr. Wenzel received her PhD from the University of Washington-Seattle where she used structure-function studies to understand specificity within the ubiquitination system.
Dr. Wenzel then trained as a postdoctoral fellow at the University of Utah where she studied how proteins from the ESCRT (Endosomal Sorting Complexes Required for Transport) pathway sever membranes to complete cell division. In particular, she focused on a cell division checkpoint known as the abscission checkpoint that synchronizes the completion of mitotic events with ESCRT-mediated membrane severing. In her studies, she identified several proteins that function with ESCRT proteins to reversibly inhibit abscission. Dr. Wenzel’s research was supported in part by an American Cancer Society Postdoctoral Fellowship.
Dr. Wenzel joined the Department of Biochemistry at the Medical College of Wisconsin in the Fall of 2020 where she continues to study signaling mechanisms that regulate cell division.
Research Interests
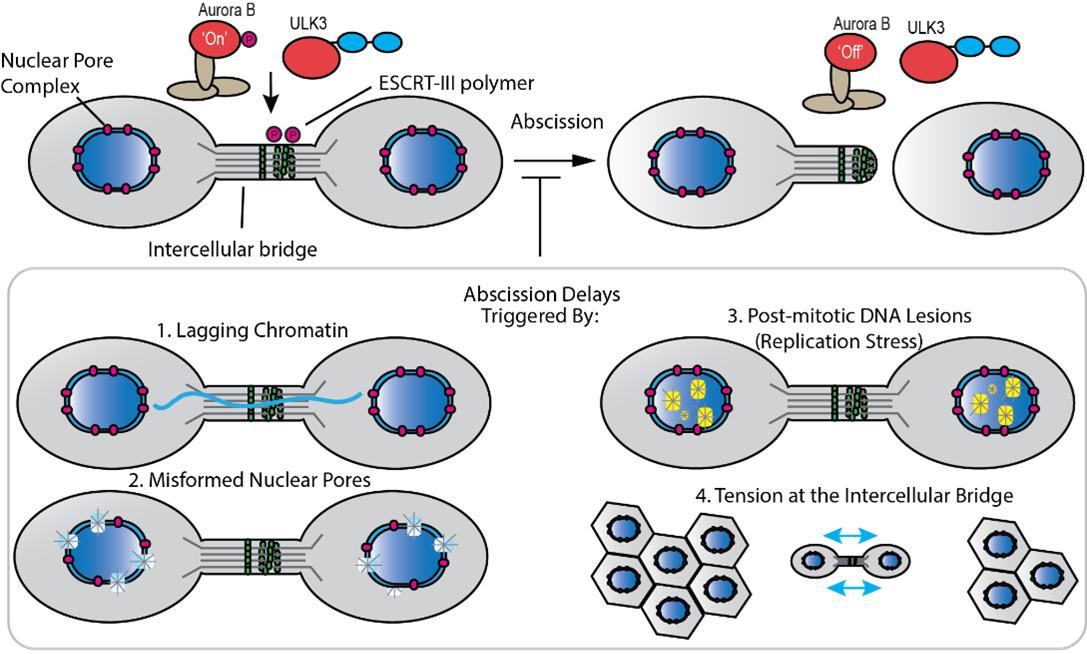
Orderly progression through cell division is regulated by a series of checkpoints which ensure the correct completion of DNA synthesis and chromosome segregation before cells can proceed to the next stage of the cell cycle. A single cell division in the presence of unsegregated chromosomes, termed lagging chromatin, can generate trademark features of cancer cells including chromosome fragmentation and micronucleation. To safeguard this process, cells have evolved a mechanism, known as the abscission checkpoint, that detects mistakes and arrests cell division at the abscission (membrane cutting) step until defects can be repaired. Our research is focused on understanding how persistent mitotic errors are sensed by the abscission checkpoint, how these errors are translated into a full abscission arrest, and finally, the signaling mechanisms that license abscission to proceed once mistakes are corrected.
Our recent studies and work from others has revealed that cells delay abscission, at least in part through inhibition of the membrane cutting machinery, known as Endosomal Sorting Complexes Required for Transport (ESCRT) proteins. One cellular strategy for ESCRT inhibition involves the sequential phosphorylation of ESCRT proteins by several key kinases known as Aurora B and ULK3. A specific focus of my lab is to understand how this phosphorylation is regulated with the goal of uncovering additional abscission protection strategies. The overarching aim of this research is to understand how healthy cells safeguard abscission and how defects in this pathway can result in diseases such as cancer. My lab uses a variety of biophysical and biochemical techniques including X-ray crystallography, fluorescence polarization and enzymatic assays to answer these questions.
If you are interested in joining the Wenzel lab, please contact Dr. Wenzel.
Publications
-
The O-GlcNAc cycling in neurodevelopment and associated diseases.
(Wenzel DM, Olivier-Van Stichelen S.) Biochem Soc Trans. 2022 Dec 16;50(6):1693-1702 PMID: 36383066 PMCID: PMC10462390 SCOPUS ID: 2-s2.0-85144232323 11/17/2022
-
(Wenzel DM, Mackay DR, Skalicky JJ, Paine EL, Miller MS, Ullman KS, Sundquist WI.) Elife. 2022 Sep 15;11 PMID: 36107470 PMCID: PMC9477494 SCOPUS ID: 2-s2.0-85138153298 09/16/2022
-
(Sadler JBA, Wenzel DM, Strohacker LK, Guindo-Martínez M, Alam SL, Mercader JM, Torrents D, Ullman KS, Sundquist WI, Martin-Serrano J.) Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):E8900-E8908 PMID: 30181294 PMCID: PMC6156662 SCOPUS ID: 2-s2.0-85053465437 09/06/2018
-
ULK3 regulates cytokinetic abscission by phosphorylating ESCRT-III proteins.
(Caballe A, Wenzel DM, Agromayor M, Alam SL, Skalicky JJ, Kloc M, Carlton JG, Labrador L, Sundquist WI, Martin-Serrano J.) Elife. 2015 May 26;4:e06547 PMID: 26011858 PMCID: PMC4475061 SCOPUS ID: 2-s2.0-84930631701 05/27/2015
-
Intrinsic disorder drives N-terminal ubiquitination by Ube2w.
(Vittal V, Shi L, Wenzel DM, Scaglione KM, Duncan ED, Basrur V, Elenitoba-Johnson KS, Baker D, Paulson HL, Brzovic PS, Klevit RE.) Nat Chem Biol. 2015 Jan;11(1):83-9 PMID: 25436519 PMCID: PMC4270946 SCOPUS ID: 2-s2.0-84927544290 12/02/2014
-
(Vittal V, Wenzel DM, Brzovic PS, Klevit RE.) Cell Biochem Biophys. 2013 Sep;67(1):103-10 PMID: 23709311 PMCID: PMC3758794 SCOPUS ID: 2-s2.0-84883447618 05/28/2013
-
(Skalicky JJ, Arii J, Wenzel DM, Stubblefield WM, Katsuyama A, Uter NT, Bajorek M, Myszka DG, Sundquist WI.) J Biol Chem. 2012 Dec 21;287(52):43910-26 PMID: 23105106 PMCID: PMC3527974 SCOPUS ID: 2-s2.0-84871586331 10/30/2012
-
Following Ariadne's thread: a new perspective on RBR ubiquitin ligases.
(Wenzel DM, Klevit RE.) BMC Biol. 2012 Mar 15;10:24 PMID: 22420831 PMCID: PMC3305615 SCOPUS ID: 2-s2.0-84858135252 03/17/2012
-
UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids.
(Wenzel DM, Lissounov A, Brzovic PS, Klevit RE.) Nature. 2011 Jun 02;474(7349):105-8 PMID: 21532592 PMCID: PMC3444301 SCOPUS ID: 2-s2.0-79957949190 05/03/2011
-
E2s: Structurally economical and functionally replete
(Wenzel DM, Stoll KE, Klevit RE.) Biochemical Journal. 1 February 2011;433(3):535 SCOPUS ID: 2-s2.0-78751498823 02/01/2011
-
E2s: structurally economical and functionally replete.
(Wenzel DM, Stoll KE, Klevit RE.) Biochem J. 2011 Jan 01;433(1):31-42 PMID: 21158740 PMCID: PMC3118098 SCOPUS ID: 2-s2.0-79551493745 12/17/2010

