Andreas Beyer Lab

Andreas M. Beyer, PhD

Curriculum Vitae (PDF)
Twitter: @BeyerLab
abeyer@mcw.edu
Andreas M. Beyer is a professor of medicine and physiology at the Medical College of Wisconsin and the co-director of the basic and translational research program in cardio-oncology.
During his training in genetics and physiology, he has gained detailed expertise in generating and evaluating novel approaches in genetics, molecular biology and physiology. In his time spent in the lab he performs experimental troubleshooting involving video microscopy, fluorescent microvascular imaging, generation of genetic rodent models, physiological evaluation of in vivo vascular function and blood pressure. With the support of this research group and important local and national collaborators, the Beyer lab is using live human tissues to address important questions in vascular biology that will lead to clinically relevant findings and drive further exploration of mechanism in rodent models. His lab hopes that clinically relevant data from human tissues will enable a detailed mechanistic understanding of disease that can then be used to develop novel therapeutics and translate both diagnostics and therapies themselves to the clinic.
Lab Projects
Current Members

Laura Norwood Toro
Research Scientist II
lnorwood@mcw.edu
Laura Norwood Toro is a research scientist in the Andreas Beyer Lab. Her primary responsibility is to explore the effect of chemotherapy on cardiovascular outcomes in coronary circulation and vascular endothelium. One focus of her studies is to investigate the functions of telomerase in the nucleus versus the mitochondria. Her background is in cell biology and molecular biology.

Steve Hammond, PhD
Postdoctoral Researcher
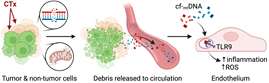
Steve Hammond is a postdoctoral researcher in the Beyer lab. Prior to his start at MCW, he earned his doctorate from Kansas State University where he investigated the adverse cardiovascular consequences of 5-fluorouracil chemotherapy and modalities to alleviate their occurrence. Steve is most interested in better understanding the mitochondrial contributions to cardiovascular function in health in disease. He is currently studying the role of mitochondrial signaling in the development of anticancer therapy induced microvascular dysfunction. Specifically, his primary projects aim to elucidate the role of tumor derived circulating factors and alterations in mitochondrial calcium signaling in the development of microvascular pathology during anti-cancer treatment.

Erin Birch
Research Technologist II
ebirch@mcw.edu
Erin Birch is a research technologist in Dr. Beyer and Dr. Zhang’s labs. Erin has experience researching autoimmune diseases, chronic kidney disease, and cardiovascular disease. She is working with Dr. Beyer’s lab to provide support with several projects. Erin also contributes to the analysis of microvascular function in patients with coronary artery disease, COVID-19, and healthy patients. In her free time, Erin enjoys traveling and adventuring in the Rocky Mountains.

Lukas Brandt
Research Assistant
lbrandt@mcw.edu
Lukas Brandt is a graduate student in Dr. Beyer's lab. Prior to starting at MCW's physiology program, he received his bachelor's degree from the University of Applied Science Bingen, Germany with a major in biotechnology. He received additional training in a Molecular Biology master program at the Goethe University Frankfurt, Germany before pursuing his education in physiology at MCW. His research interests are whole organ physiology with a focus on cardiovascular pathology in response to clinical used anti-cancer therapy. Lukas aims to utilize rodent models to investigate physiological and molecular changes that lead to cardiovascular disease.

Cristhian Gutierrez Huerta
Research Assistant Graduate School
cgutierrez@mcw.edu
Cristhian Gutierrez Huerta is a graduate MSTP student in the Beyer/Gutterman Lab. He graduated with a BS in Applied Mathematics and Biological Sciences from the University of California, Merced in 2018. He then completed a 2-yr post-baccalaureate research fellowship at the National Heart, Lung, and Blood Institute. In the Beyer/Gutterman group, he will begin work on identifying the role of mitochondrial fission/fusion on microvascular endothelial function and its relationship to coronary artery disease progression.

Yoshinori Nishijima
Research Associate
ynishijima@mcw.edu
My current research is focused on understanding the transient receptor potential vanilloid 4 channels to angiotensin II-induced impairment of vasodilation in animals and humans. I am also interested in the role of vascular smooth muscle potassium channels in hydrogen peroxide-induced vasodilation in human arterioles, in health and disease.
Alumni/Former Trainees
- Karima Ait-Aissa
- Karen Clark, PhD
- Daniela Didier
- Johnathan Ebben
- Alena Hanson
- Joe Hockenberry
- Bill Hughes, PhD
- Andrew D. Kadlec, PhD
- Minhi Kang
- Todd Le
- Jasmine Linn
- Janée Terwoord
- Micaela Young
Recent Publications
-
Telomer length, integrity vs. telomerase-activity - who is to blame for heart failure?
(Kleinbongard P, Beyer AM.) Cardiovasc Res. 2026 Feb 03 PMID: 41634935 02/04/2026
-
Autophagy modulates the mechanism of flow-mediated dilation upstream of telomerase.
(Hughes WE, Hader SN, Astbury K, Brandt L, Ait-Aissa K, Gutterman DD, Beyer AM.) Basic Res Cardiol. 2025 Dec;120(6):1131-1140 PMID: 41238787 PMCID: PMC12680695 SCOPUS ID: 2-s2.0-105021839661 11/15/2025
-
(Venkatasubramanian R, Mahoney SA, Hutton DA, VanDongen NS, Brunt VE, Greenberg NT, Longtine AG, Brandt L, Beyer AM, Melov S, Rossman MJ, Seals DR, Clayton ZS.) Am J Physiol Heart Circ Physiol. 2025 Dec 01;329(6):H1672-H1683 PMID: 41143747 PMCID: PMC12704655 SCOPUS ID: 2-s2.0-105024255414 10/27/2025
-
(Terwoord JD, Norwood Toro LE, Hader SN, Hammond ST, Hockenberry JC, Linn J, Vazirabad IY, Kong AL, Kriegel AJ, Liu Z, Kivelä RM, Murtagh G, Gutterman DD, Beyer AM.) JCI Insight. 2025 Nov 24;10(22) PMID: 41026534 PMCID: PMC12643535 SCOPUS ID: 2-s2.0-105022722049 09/30/2025
-
(Bikomeye JC, McGinley EL, Zhou Y, Tarima S, Kwarteng JL, Beyer AM, Yen TWF, Winn AN, Beyer KMM.) JACC Adv. 2025 Sep;4(9):102069 PMID: 40829368 PMCID: PMC12396096 SCOPUS ID: 2-s2.0-105013235004 08/20/2025
-
(Do Couto N, Hidde M, Grigoriadis G, Sparapani R, Durand M, Widlansky M, Jankowski C, Berendt M, Canales B, Golus S, Norwood Toro LE, Laud P, Kong A, Hoskins K, Lewandowski D, Phillips SA, Gutterman DD, Kriegel AJ, Beyer KMM, Beyer AM, Stolley M.) Cardiooncology. 2025 Aug 21;11(1):75 PMID: 40841694 PMCID: PMC12369258 SCOPUS ID: 2-s2.0-105014033548 08/22/2025
-
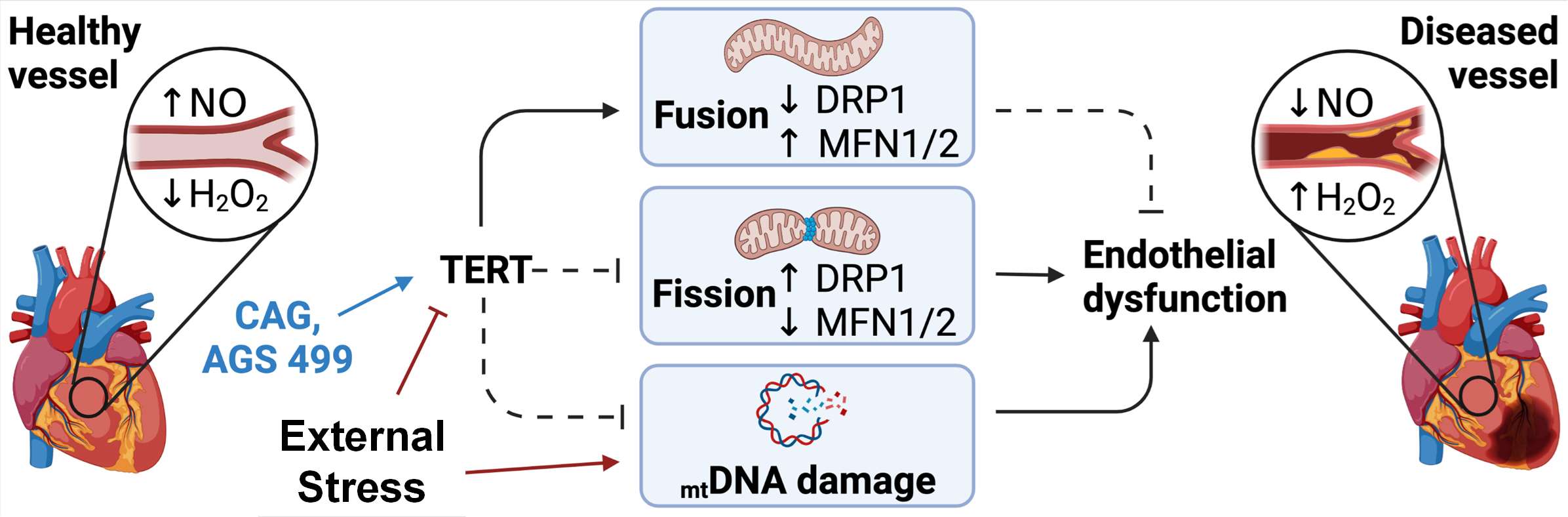
Endothelial TERT drives microvascular phenotype associated with coronary artery disease.
(Birch EC, Nishijima Y, Hader SN, Beyer AM.) Am J Physiol Heart Circ Physiol. 2025 Jul 01;329(1):H267-H270 PMID: 40514200 PMCID: PMC12233018 SCOPUS ID: 2-s2.0-105009755634 06/14/2025
-
(Nishijima Y, Hader SN, Birch EC, Chen Y, Widlansky ME, Beyer AM.) Cardiovasc Res. 2025 May 23;121(5):699-701 PMID: 39943809 SCOPUS ID: 2-s2.0-105006564276 02/13/2025
-
(Bikomeye JC, Tarima S, Zhou Y, Kwarteng JL, Beyer AM, Yen TWF, Winn AN, Beyer KMM.) Health Place. 2025 May;93:103460 PMID: 40187121 SCOPUS ID: 2-s2.0-105001825932 04/06/2025
-
Autophagy modulates the mechanism of flow-mediated dilation upstream of telomerase
(Hughes WE, Hader SN, Astbury K, Brandt L, Ait-Aissa K, Gutterman DD, Beyer AM.) Basic Research in Cardiology. December 2025;120(6):1131-1140 SCOPUS ID: 2-s2.0-105021839661 12/01/2025
-
(Bikomeye JC, McGinley EL, Zhou Y, Tarima S, Kwarteng JL, Beyer AM, Yen TWF, Winn AN, Beyer KMM.) Cancer Surviv Res Care. 2025;3(1) PMID: 40881314 PMCID: PMC12382361 09/01/2025
-
UBR1 Promotes Sex-Dependent ACE2 Ubiquitination in Hypertension.
(Elgazzaz M, Lakkappa N, Berdasco C, Mohan UP, Nuzzo A, Restivo L, Martinez A, Scarborough A, Guidry JJ, Sriramula S, Xu J, Daoud H, Mendiola Plá MA, Bowles DE, Beyer AM, Mauvais-Jarvis F, Yue X, Filipeanu CM, Lazartigues E.) Hypertension. 2025 Jan;82(1):84-95 PMID: 39601126 PMCID: PMC11655255 SCOPUS ID: 2-s2.0-85213490001 11/27/2024