Innovative Neuroimaging Technique Could Improve Outcomes for Glioblastoma Patients
Glioblastoma is a devastating and incurable brain cancer that affects thousands of individuals worldwide each year. With a prognosis that is universally fatal, the fight against this cancer remains one of the most challenging in modern medicine. However, recent advances in neuroimaging may offer new hope for better tumor detection, which may improve treatment and patient outcomes. Peter S. LaViolette, PhD '11, MS, holder of the Robert C. Olson, MD, Professorship in Radiology, vice chair of radiology research, and director of the Radiology Quantitative Imaging Laboratory at the Medical College of Wisconsin (MCW), is at the forefront of this transformation.

Thanks to a five-year, $3.3 million R01 grant from the National Institutes of Health (NIH) and National Cancer Institute (NCI), Dr. LaViolette and his team are poised to refine and validate cutting-edge imaging techniques that could revolutionize the way doctors treat gliomas, the group of brain tumors that includes glioblastoma. The MCW team includes Jennifer Connelly, MD (Neurology), Max Krucoff, MD (Neurosurgery), Samuel Bobholz, PhD (Radiology), Anjishnu Banerjee, PhD (Biostatistics/Data Science), E. Kelly Mrachek, MD (Pathology), and Andrew Nencka, PhD (Radiology).
The grant, titled “Radiopathomic Modeling of Glioma Heterogeneity Throughout a Patient’s Disease Trajectory,” is designed to advance an innovative approach known as radiopathomic mapping. This method integrates medical imaging with pathological data from both autopsy and biopsy samples to track the infiltration and progression of gliomas – information that has traditionally been difficult to capture using conventional MRI alone. Dr. LaViolette’s work, which combines advanced imaging technology with artificial intelligence (AI), promises to provide a more accurate and comprehensive understanding of how tumors evolve over time, leading to better, more targeted treatments for patients.
Radiopathomic Mapping: Advancing Tumor Detection
Glioma brain tumors are notoriously difficult to treat due to their infiltrative nature, meaning they often spread beyond what standard imaging can detect. Traditional MRIs can miss these microscopic tumor cells, leading to incomplete assessments of the tumor’s true extent. Radiopathomic mapping, however, aims to address this limitation by providing a more nuanced view of how the tumor infiltrates healthy brain tissue.
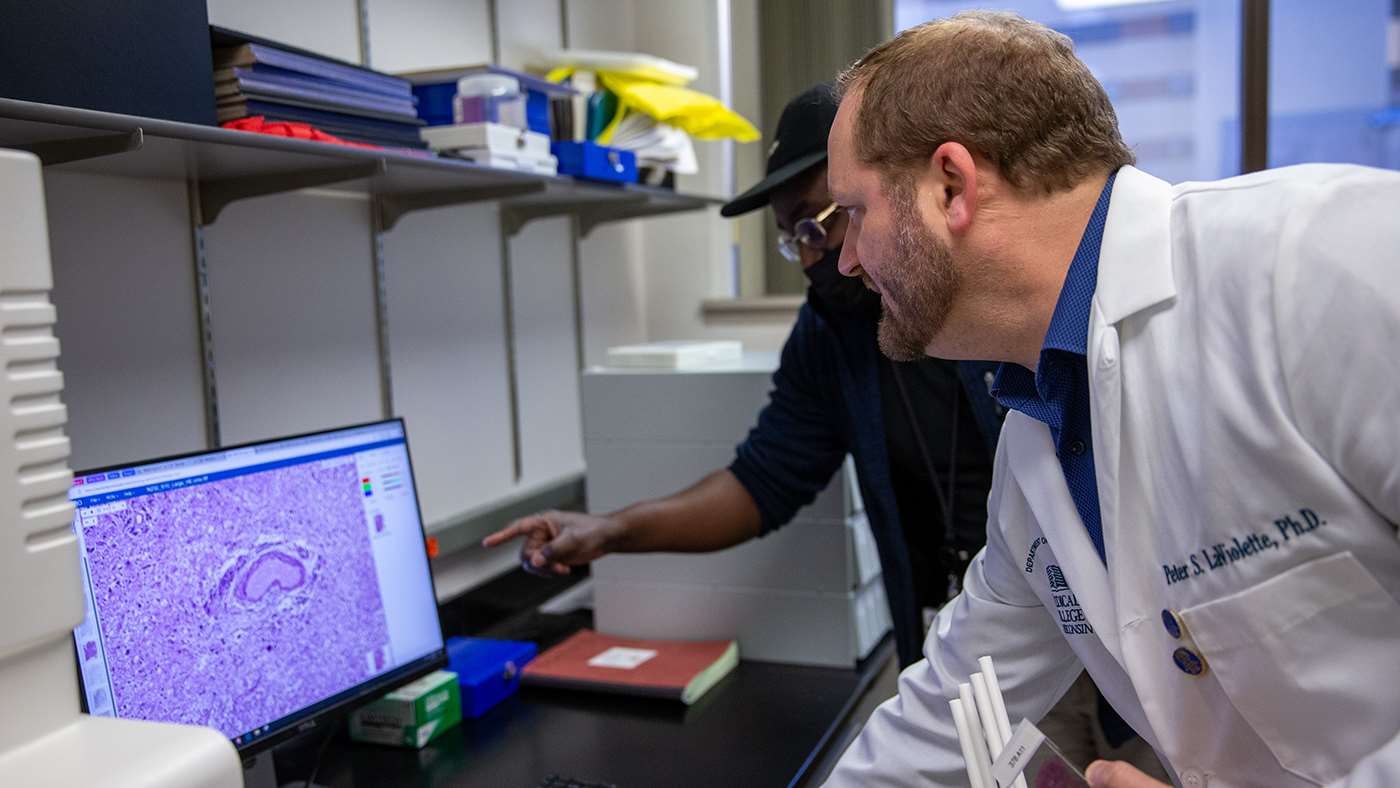
Through this technique, Dr. LaViolette’s team integrates detailed tissue samples with MRI scans to create what are known as radiopathomic maps of histo-morphometric features such as tumor cell density. These maps provide a visual representation of the tumor’s microscopic spread, potentially allowing doctors to detect tumor regions that were previously invisible. By identifying these hidden tumor cells, doctors can more accurately target treatment areas, ensuring that the full extent of the tumor is addressed.
“The infiltrative nature of gliomas makes it difficult to define and treat the full extent of the tumor using conventional MRI. Accurate methods for delineating the true extent of the tumor are desperately needed for directing treatments and evaluating their effectiveness,” Dr. LaViolette says.
Cross-Institution Collaboration: A Combined Approach for Better Brain Tumor Treatment
The project also highlights a collaborative effort between Dr. LaViolette’s team at MCW and the team under Janine Lupo, PhD, at the University of California, San Francisco (UCSF). Both institutions have developed their own radiopathomic techniques, using data from different sources – autopsy samples at MCW and biopsy cores at UCSF. This NIH/NCI grant will allow the teams to compare and combine their models, validating their effectiveness while refining the algorithms for broader application.
“What’s exciting about this project is that we are bringing together two complementary datasets,” Dr. LaViolette says. “UCSF’s biopsy cores represent a more ‘pure’ cohort of glioblastoma patients in the pre-treatment stage, while our autopsy samples reflect tumors that have undergone multiple rounds of treatment. The combination of these two datasets will help us understand how tumors behave at different stages of the disease and refine our models accordingly.”
This partnership also involves integrating clinical data and deep learning techniques into the radiopathomic models. By incorporating variables like the timing of MRI scans, treatment history and patient prognosis, Dr. LaViolette’s team hopes to create a more robust model that accounts for how gliomas evolve over time. Additionally, the use of deep learning will allow the team to refine their algorithms further, making the models more accurate and adaptable to different patient scenarios.
Multisite Validation: Bringing Neuroimaging Research to a Clinical Setting
The final aim of the NIH project is to prospectively validate the radiopathomic maps in a clinical setting. This step is crucial for determining whether the new models can truly guide clinical decision-making. As part of this clinical validation, the team plans to conduct a multisite trial to test how well the radiopathomic maps can guide surgeons in identifying the most invasive regions of the tumor for biopsy.
“The goal is to prove in a prospective setting that it actually works and that our imaging is truly something that can guide a doctor’s decision-making when it comes to treating these brain tumors,” Dr. LaViolette says.
By giving surgeons a more precise map of tumor infiltration, the team hopes to improve tumor targeting and potentially reduce the need for invasive surgeries. In cases where surgery might risk damage to critical brain functions, radiopathomic mapping could offer an alternative approach by guiding radiation therapy to more accurately target the tumor.
The Future of Glioma Treatment
The long-term goals of Dr. LaViolette’s research are nothing short of transformative. By advancing radiopathomic mapping techniques, his team aims to provide clinicians with noninvasive tools that can guide treatment planning, monitor responses to treatment and improve patient outcomes. With continued refinement, these models could help personalize treatment for glioblastoma patients, offering a more targeted approach that addresses the unique characteristics of each tumor. “Ultimately, we want to enable better tracking of glioblastoma,” Dr. LaViolette says. “By refining our models and expanding our dataset, we can improve the lives of patients and offer them a fighting chance against this devastating illness.”



