MCW Ocular Gene Therapy Laboratory Research
Designing the Next Generation of Gene Therapy Treatments
View Our Publications

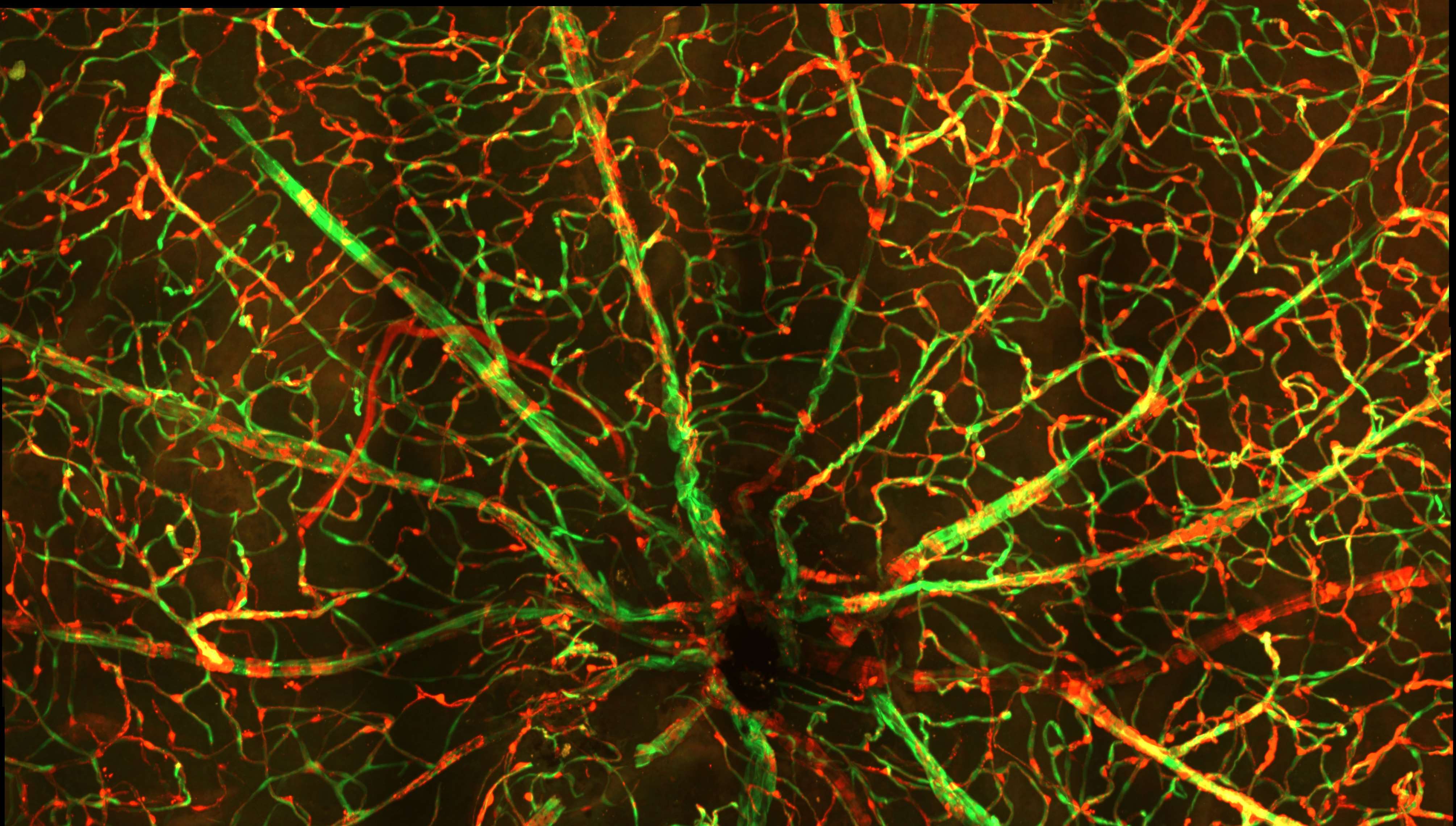
Development of gene therapy treatments to prevent vascular dysfunction in diabetic retinopathy
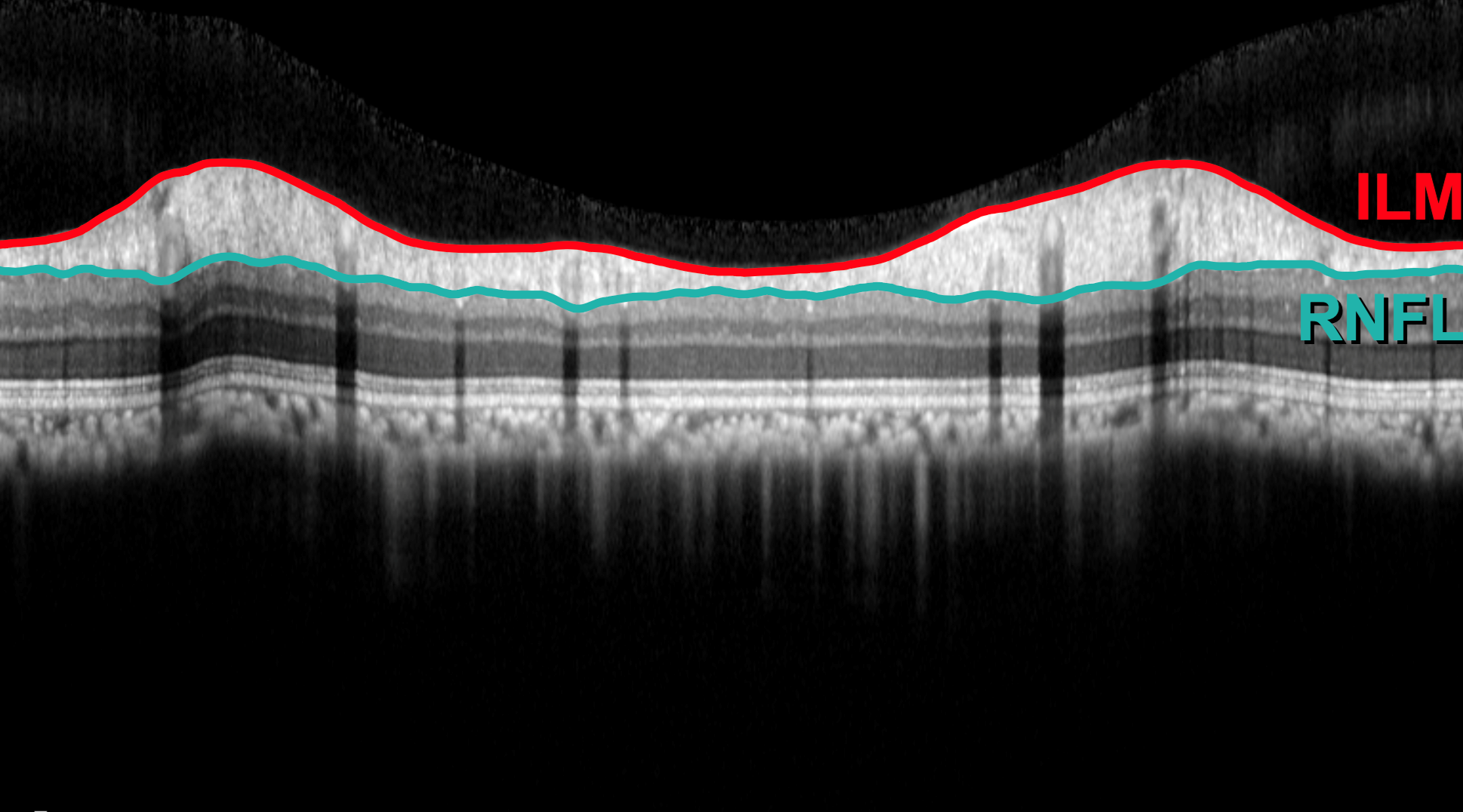
Development of gene therapy technologies for the treatment of primary open angle glaucoma (POAG)
POAG is one of the leading causes of blindness in the working-age population, affecting approximately 57.5 million worldwide. In the majority of glaucoma patients, an imbalance between the amount of fluid (aqueous) entering and leaving the eye causes increased intra-ocular pressure (IOP) and the death of retinal ganglion cells, specialized neurons within the eye that make up the optic nerve and are responsible for transmitting visual information from the retina to the brain. Whilst existing pharmacological and surgical treatments aimed at lowering IOP can be extremely effective, they place a high medical burden on the patient, usually requiring adherence to a life-long daily treatment regimen of eye drops to relieve pressure. The OGTL is currently pursuing an exciting alternative strategy to traditional pharmacological approaches capable of permanently and safely lowering IOP in glaucomatous eyes following a single gene therapy treatment; the early pre-clinical stages of this work have shown promising dose-dependent reduction in pressure and have been generously supported through intra-mural funding from the MCW Office of Technology and Development (OTD).
In addition to developing long-acting treatments for glaucoma we are investigating methodologies to non-invasively deliver new genetic material to cells of the cornea using a contact lens-based delivery system in work supported but a Shaffer Award to Dr. Lipinski (PI) from the Glaucoma Research Foundation (GRF).
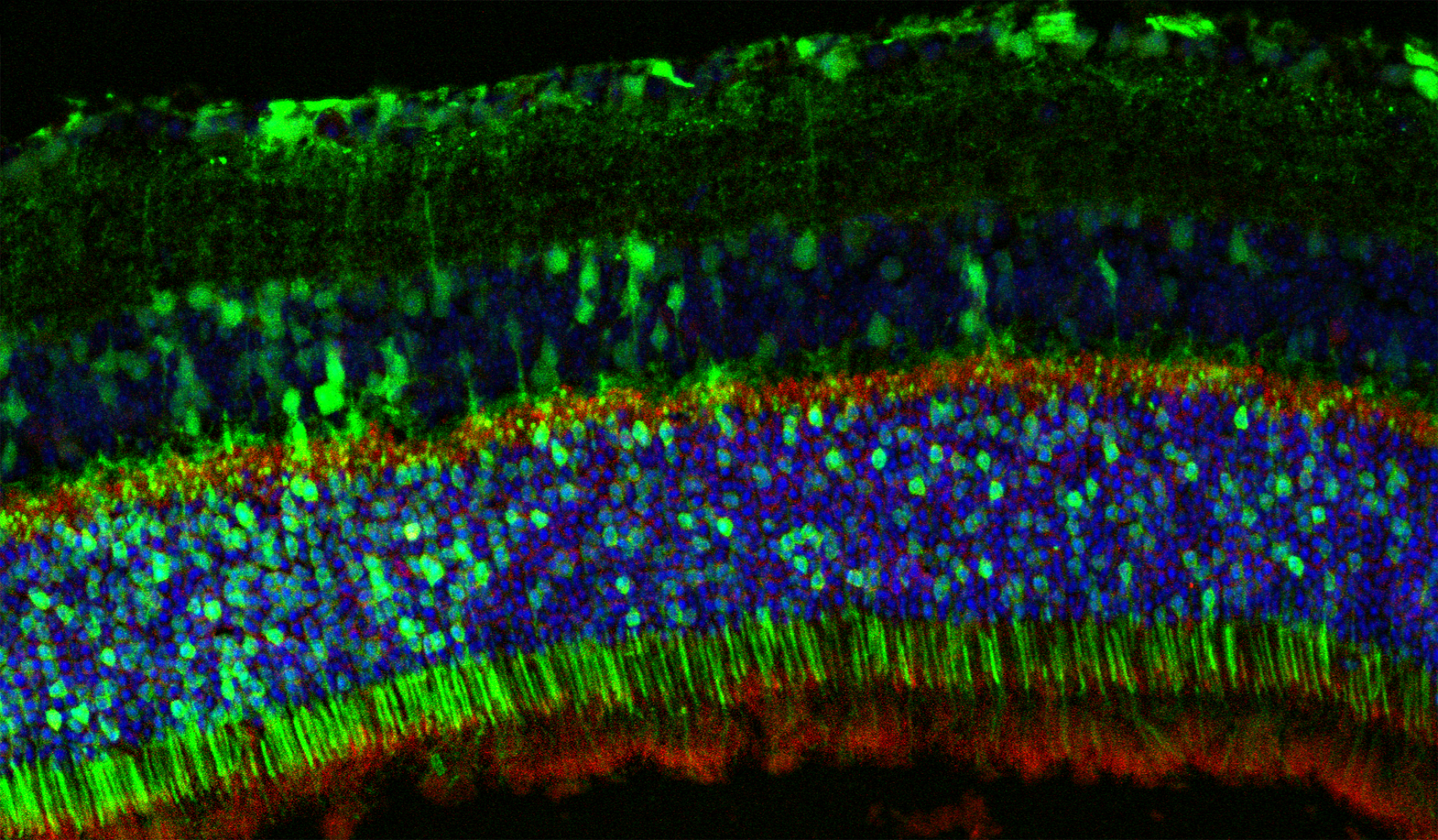
Evaluation of gene therapy treatments for rare inherited retinal disease
Inherited retinal diseases collectively affect 1 in 4000 individuals worldwide and are characterized by a progressive loss of photoreceptor cells, leading to blindness that is both irreversible and untreatable. In the majority of patients, rod photoreceptors, which provide for nighttime vision, degenerate first due to an underlying genetic abnormality, followed by the secondary loss of genetically normal cone photoreceptors. The OGTL, funded through a Foundation for Fighting Blindness Individual Investigator Award (TA-NMT-0618-0739) to Dr. Lipinski (PI) is investigating whether over-expression of a group of proteins called proteolysis inhibitors is able to physically prevent cone photoreceptors from being broken down in degenerative disease.
In addition to working on the development of a gene-independent strategies to protect against cone photoreceptor degeneration, we are also working on collaborative projects aimed at developing gene-specific therapies for hyper-rare systemic diseases that have an ocular phenotype, including Farber lipogranulomatosis (Collaborator: Dr. Jeffery Medin, MCW) and gyrate atrophy (Collaborators: Drs. Mandeep Singh and David Valle, Johns Hopkins), conditions with fewer than 500 reported cases between them.