Genomics, Genetics and Epigenetics Laboratory

Featured Family Story: A Piece of the Puzzle - Finding a Name for the Unknown
Research Areas
Genetic studies of Human Ocular Disorders
Ocular development is a complex process requiring carefully orchestrated differentiation of cells. Study of the genetic causes of pediatric/early onset conditions provides important insight into the mechanisms of normal development and disease. For affected families, identification of a genetic cause provides more accurate diagnosis and medical management.


Developmental Genomics Program
Many genes important in early development are active in many parts of the body. Disruption of one of these genes can cause multiple congenital anomaly (MCA) syndromes. The current diagnosis rate for exome sequencing in MCA syndromes varies from 15-50% in different settings, indicating that additional causes remain to be identified. Through analysis of samples from affected families as well as work in zebrafish and iPSC cell lines, we aim to expand understanding of known developmental genes and identify new genes important in early development.

Get Involved
Support the Genomics, Genetics, and Epigenetics (GGE) Laboratory
The GGE Laboratory could not exist without the generosity and support of federal agencies and individual donors. Funds donated to the program are used to acquire new equipment and research consumables, to provide clinically confirmed results back to participants, to enable the presentation of our research findings at scientific meetings and to fund positions for support staff. Please contact SeminaGenetics@mcw.edu with have any questions. If you are interested in supporting our program at any level please click below, select “Other” from the drop-down menu and indicate ‘Elena Semina’ to donate securely online.
Donate to the GGE Lab
Join our studies
Enrollment in our studies is open to individuals affected with relevant conditions living in the United States or Canada. We are able to enroll in-person or remotely via a phone/Zoom call with samples collected at home.Genetic Studies of Human Ocular Disorders
Developmental Genomics Program
Feedback From Our Families
Having a confirmed diagnosis has been wonderful. I can say this is what my child has and this is how we can help him.
GGE Lab Study Participant
"Very appreciative to have been included. Also, very happy with the professionalism of the staff of researchers and the quick and personal responses"
GGE Lab Study Participant
"Relief. While the number of individuals identified with this syndrome is incredibly small, the characteristics or potential other issues are minimal. The nagging worry that something may be seriously wrong and just was not identified yet is gone."
GGE Lab Study Participant
"I am happy our genetic cause has been identified so now more families can be helped."
GGE Lab Study Participant
Meet Our Team

Elena Semina, PhD
Marjorie and Joseph Heil Professor of Ophthalmology & Visual Sciences; Professor, Pediatrics; Professor, Cell Biology, Neurobiology and Anatomy

Isabelle Banke, BS
Clinical Research Coordinator II

Kailey Frank, BS
Research Technologist II

Megan Fischer
G3 Student

Justin Freestone
Graduate Student
Gary Gardner
Research Technologist I
Tierel T. Hood-Nellum
Lab Technician II

Linda Reis, MS, LCGC
Program Manager; Adjunct Instructor

Maria R. Replogle, PhD
Research Scientist I

Sarah Seese, PhD
Research Scientist I

Elena A. Sorokina, MS
Research Scientist I
Samuel Thompson
Research Technologist III
Publications
Contact Us
Genomics, Genetics, and Epigenetics (GGE) Laboratory
(414) 955-7645
seminagenetics@mcw.edu





 Ferre-Fernández JJ, Reis LM, Semina EV.
Ferre-Fernández JJ, Reis LM, Semina EV.  Reis LM, Basel D, Bitoun P, Walton DS, Glaser T, Semina EV.
Reis LM, Basel D, Bitoun P, Walton DS, Glaser T, Semina EV. 

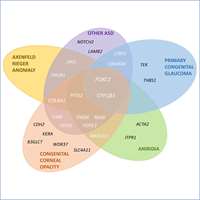


 Reis LM, Maheshwari M, Capasso J, Atilla H, Dudakova L, Thompson S, Zitano L, Lay-Son G, Lowry RB, Black J, Lee J, Shue A, Kremlikova Pourova R, Vaneckova M, Skalicka P, Jedlickova J, Trkova M, Williams B, Richard G, Bachman K, Seeley AH, Costakos D, Glaser TM, Levin AV, Liskova P, Murray JC, Semina EV.
Reis LM, Maheshwari M, Capasso J, Atilla H, Dudakova L, Thompson S, Zitano L, Lay-Son G, Lowry RB, Black J, Lee J, Shue A, Kremlikova Pourova R, Vaneckova M, Skalicka P, Jedlickova J, Trkova M, Williams B, Richard G, Bachman K, Seeley AH, Costakos D, Glaser TM, Levin AV, Liskova P, Murray JC, Semina EV. 




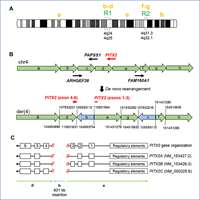
 Amlie-Wolf L, Bardakjian T, Kopinsky SM, Reis LM, Semina EV, Schneider A.
Amlie-Wolf L, Bardakjian T, Kopinsky SM, Reis LM, Semina EV, Schneider A. 



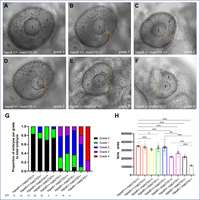
 Reis LM, Sorokina EA, Dudakova L, Moravikova J, Skalicka P, Malinka F, Seese SE, Thompson S, Bardakjian T, Capasso J, Allen W, Glaser T, Levin AV, Schneider A, Khan A, Liskova P, Semina EV.
Reis LM, Sorokina EA, Dudakova L, Moravikova J, Skalicka P, Malinka F, Seese SE, Thompson S, Bardakjian T, Capasso J, Allen W, Glaser T, Levin AV, Schneider A, Khan A, Liskova P, Semina EV. 



 Reis LM, Basel D, McCarrier J, Weinberg DV, Semina EV.
Reis LM, Basel D, McCarrier J, Weinberg DV, Semina EV. 
