Welcome to the Nakagawa Lab

Our Mission
Current Projects
Hypertension: The Silent Killer
Despite significant progress, cardiovascular (CV) diseases are still the leading cause of death globally. One of the main risk factors for CV disease is elevated blood pressure (BP) or hypertension (HT). High BP is typically managed with a wide variety of drugs. However, a vast number of patients with HT do not achieve desirable BP with conventional therapeutic options. Notably, patients who exhibit resistant HT are associated with autonomic imbalance and therefore, novel strategies to ameliorate sympathoexcitation and restore the depressed vagal parasympathetic tone (renal nerve ablation, vagus nerve stimulation, carotid baroreflex therapy, acetylcholinesterase inhibitors, etc.) are now recognized as potential therapeutic options for diseases beyond HT.
Thus, our laboratory’s main focus is to understand the fundamental neural mechanisms that control BP and the autonomic nervous system. We believe that our findings might contribute to developing new alternative therapies to treat HT.
(Fig. 1 - right)
The Brain RAS: A Fundamental Mechanism of Blood Pressure Control
The renin-angiotensin system (RAS) is one of the most important mechanisms of BP control and fluid and electrolyte homeostasis. As shown in Figure 2, the activation of the RAS requires a two-step enzymatic process to generate Angiotensin II, an octapeptide that exerts potent pressor effects through angiotensin type 1 receptors (AT1R)-mediated mechanism. Importantly, renin plays a critical role in the regulation of the RAS since it is the rate-limiting enzyme of this biosynthetic cascade. Besides the classical RAS found in the circulating system, the existence of an unconventional RAS that operates locally in specific tissues, such as the brain, has been proposed.
Although the critical role of the local activation of the RAS in the brain to control BP regulation, hydromineral balance, autonomic function, and metabolism is well accepted it remains unclear how and where angiotensin (ANG)-II, the main bioactive component of the RAS, is generated within the brain; Surprisingly, whether renin, the rate-limiting enzyme of ANG-II biosynthesis, is expressed and necessary for the cleavage of angiotensinogen (AGT) to generate ANG-I in the brain remains controversial.
(Fig. 2 - left)
Unraveling a Scientific Mystery That Has Persisted for 5 Decades: the Discovery of Renin-Expressing Neurons in the Nucleus Ambiguus
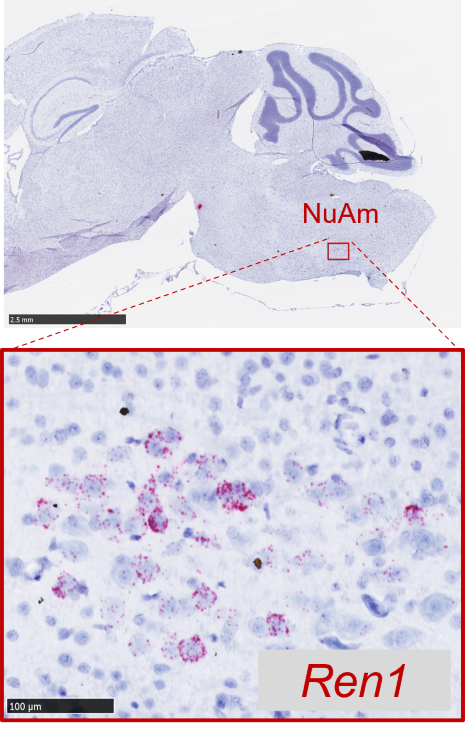
For many years, conventional techniques that are normally used to detect the abundant amounts of renin in plasma and kidneys have been used in an attempt to detect renin in whole brain tissue homogenates. But these methods lack sensitivity, specificity, and especially the anatomical precision to detect neurons that produce extremely low amounts of renin. Thus, these questionable reports raised a misconception that renin is not expressed in the brain. Our team has mastered key technology from neuroscience and acquired expertise in neuroanatomy to address this important question with the appropriate tools and techniques. These advanced techniques and new knowledge helped us to obtain convincing evidence for the expression of renin in cholinergic neurons within the brainstem, specifically, the nucleus ambiguus (NuAm), which is a relatively understudied brainstem region in the field of hypertension (Fig. 3).
This exciting discovery is critical since the existence of a select population of neurons expressing renin incorporates new evidence that ANG might operate as a neurotransmitter in the brain. There are two main theories about this topic: a) the volume transmission hypothesis where ANG peptides are generated in the extracellular space acting as neurohormones, and b) the wiring transmission hypothesis where ANG generated by neurons acts as a neurotransmitter itself. Although several studies support the second possibility, the lack of evidence that renin is expressed in neurons has been the major limitation in defining ANG as a neurotransmitter. Thus, the identification of the renin-neurons in the NuAm could be an important finding to solve this critical question. Cellular, molecular, and neurobiological characterization of renin-expressing neurons will provide key information to understand how the RAS operates in the brain and ensure definitive evidence of the functional role of renin in the brain.
(Fig. 3)
New Advances: Characterizing the Novel Renin-Expressing Neurons Using State-of-the-Art Technology
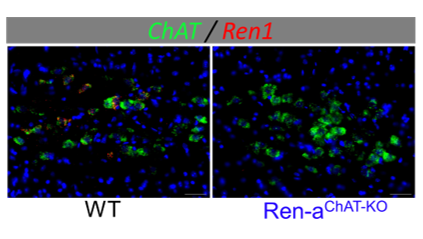
(Fig. 4)
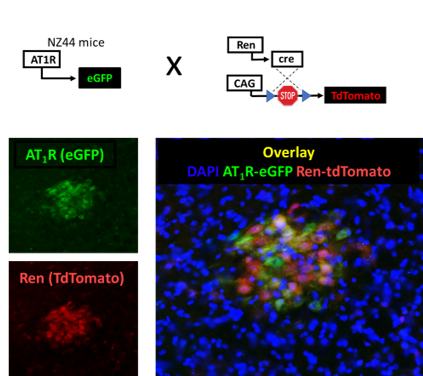
(Fig. 5)
Meet Our Team

Pablo Nakagawa, PhD
Assistant Professor

Mina Ghobrial
G3 Student

Ana Hantke Guixa, BS
Research Technologist I

Katie Kaminski
Graduate Student

Haruka Okabe, MS
Research Technologist I
Publications
-
(Golosova D, Kumar G, Chaihongsa N, Reho JJ, Lu KT, Brozoski DT, Wackman KK, Lawton SBR, Muskus PC, Lin BL, Grobe JL, Nakagawa P, Sigmund CD.) Hypertension. 2025 Nov;82(11):e348 PMID: 41091907 SCOPUS ID: 2-s2.0-105018997214 10/15/2025
-
Physiological and Molecular Implications of Angiotensinergic Signaling in the Brainstem.
(Ghobrial M, Gomez RA, Grobe JL, Sigmund CD, Nakagawa P.) Endocrinology. 2025 Sep 08;166(10) PMID: 40845152 PMCID: PMC12418094 SCOPUS ID: 2-s2.0-105015546789 08/22/2025
-
Cullin-3 regulates the renal baroreceptor machinery that controls renin gene expression.
(Golosova D, Kumar G, Lu KT, Muskus Veitia PC, Hantke Guixa A, Wackman KK, Fekete EM, Brozoski DT, Grobe JL, Sequeira-Lopez MLS, Gomez RA, Nakagawa P, Sigmund CD.) JCI Insight. 2025 Aug 08;10(15) PMID: 40627460 PMCID: PMC12333948 SCOPUS ID: 2-s2.0-105013217775 07/08/2025
-
(Fekete ÉM, Gomez J, Ghobrial M, Kaminski K, Muskus PC, Boychuk CR, Hantke Guixa A, Vazirabad I, Xie M, Ganiyu A, Golosova D, Mathieu NM, Wang YB, Lu KT, Wackman KK, Brozoski DT, Mouradian GC, Hodges MR, Segar JL, Grobe JL, Sigmund CD, Nakagawa P.) Hypertension. 2025 Feb;82(2):282-292 PMID: 39618396 PMCID: PMC11735315 SCOPUS ID: 2-s2.0-85210962734 12/02/2024
-
(Pasos J, Wagner VA, Lawton SBR, Madison AM, Nandani K, Mathieu NM, Grobe CC, Reho JJ, Freudinger BP, Sherman AJ, Brozoski DT, Morselli LL, Burnett CML, Segar JL, Nakagawa P, Sigmund CD, Grobe JL.) Iscience. 18 July 2025;28(7) SCOPUS ID: 2-s2.0-105007661529 07/18/2025
-
(Golosova D, Kumar G, Chaihongsa N, Reho JJ, Lu KT, Brozoski DT, Wackman KK, Lawton SBR, Muskus PC, Lin BL, Grobe JL, Nakagawa P, Sigmund CD.) Hypertension. 1 July 2025;82(7):1208-1220 SCOPUS ID: 2-s2.0-105005164668 07/01/2025
-
(Ziegler AA, Lawton SBR, Fekete EM, Brozoski DT, Wagner VA, Grobe CC, Sigmund CD, Nakagawa P, Grobe JL, Segar JL.) Am J Physiol Regul Integr Comp Physiol. 2025 Jan 01;328(1):R109-R120 PMID: 39548798 PMCID: PMC11905802 SCOPUS ID: 2-s2.0-85213215949 11/16/2024
-
(Lawton SBR, Wagner VA, Nakagawa P, Segar JL, Sigmund CD, Morselli LL, Grobe JL.) Hypertension. 2024 Nov;81(11):2209-2217 PMID: 39315447 PMCID: PMC11483214 SCOPUS ID: 2-s2.0-85205521844 09/24/2024
-
(Mathieu NM, Tan EE, Reho JJ, Brozoski DT, Muskus PC, Lu KT, Wackman KK, Grobe JL, Nakagawa P, Sigmund CD.) Hypertension. 2024 Jun;81(6):1332-1344 PMID: 38629290 PMCID: PMC11096025 SCOPUS ID: 2-s2.0-85193429264 04/17/2024
-
(Reho JJ, Muskus PC, Bennett DM, Grobe CC, Burnett CML, Nakagawa P, Segar JL, Sigmund CD, Grobe JL.) Am J Physiol Regul Integr Comp Physiol. 2024 Mar 01;326(3):R242-R253 PMID: 38284128 PMCID: PMC11213288 SCOPUS ID: 2-s2.0-85186271298 01/29/2024
-
(Ziegler AA, Lawton SBR, Grobe CC, Reho JJ, Freudinger BP, Burnett CML, Nakagawa P, Grobe JL, Segar JL.) Am J Physiol Regul Integr Comp Physiol. 2023 Nov 01;325(5):R576-R592 PMID: 37720996 PMCID: PMC10866575 SCOPUS ID: 2-s2.0-85174752582 09/18/2023
-
(Mathieu NM, Fekete EM, Muskus PC, Brozoski DT, Lu KT, Wackman KK, Gomez J, Fang S, Reho JJ, Grobe CC, Vazirabad I, Mouradian GC Jr, Hodges MR, Segar JL, Grobe JL, Sigmund CD, Nakagawa P.) Function (Oxf). 2023;4(5):zqad043 PMID: 37609445 PMCID: PMC10440998 SCOPUS ID: 2-s2.0-85168790693 08/23/2023








